| |
 |
| |
| Lithium-Air Batteries with split Oxygen Harvesting and Redox Processes (LABOHR) |
|
Prof. John Owen
Univ. Southampton, UK
J.R.Owen@soton.ac.uk
|
| |
The LABOHR project1 is a 3 year collaborative project funded by the European Union, in an effort to demonstrate an electric vehicle with a range of 500 km. This require-ment translates into a target specification of 500 Wh per kg of the battery that would serve as the sole power store for the vehicle.
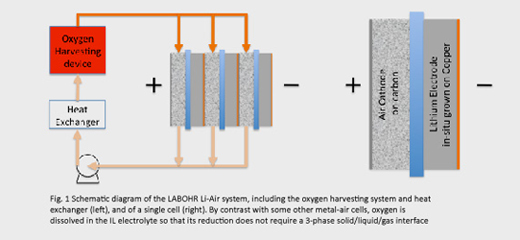
Several designs of lithium –air cell have been proposed to meet this challenge, and a distinctive feature of the LABOHR project is the concept of physically separating the process of absorbing oxygen from air into a liquid electrolyte from the electrochemical reaction, as illustrated schematically in Fig. 1.
|
 |
|
The electrolytes are chosen from ionic liquids containing pyridynium-based cations, that are believed to give better stability than the organic carbonates to the aggressive radical species involved in oxygen reduction. The ionic liquids will also be required to allow efficient mass transport of oxygen from the harvesting site to the electrode. Interfacial transport at the air interface and the electrode surface will be limited to some extent by permeability of oxygen in the ionic liquid, and this presentation will describe the evaluation of oxygen solubility and diffusion as a function of temperature within the expected operating range, using electrochemical cells that mimic the operation of the battery while accurately defining the diffusion geometry. We will also consider alternative oxygen carriers in the form of additives dissolved in the electrolyte. Other major aspects covered in this study are the interfacial reactions, the nature of the reduction products and their re-oxidation during charging the battery.
|
|
| [1] EU-LABOHR project (FP7-2010-GC-Electrochemical-Storage 265971). |
|
| < volver |
|
| |