|
|
|
| |
 |
| |
| Unlocking the Chemistry of the Lithium-Oxygen Cell |
|
Laurence J. Hardwick
Liverpool University, UK
laurence.hardwick@liv.ac.uk
|
| |
To satisfy the energy storage needs of society in the long-term, an advance in battery energy density is required. The non-aqueous lithium-oxygen (Li-O2) battery is one of the emerging opportunities available for enhanced energy storage [1]. Unlike a conventional battery where the reagents are contained within the cell, the Li-O2 cell uses oxygen from the atmosphere.
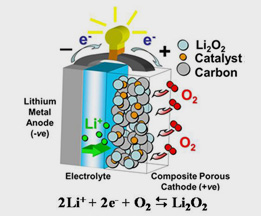
The Li-O2 cell can be thought of as a battery-fuel cell hybrid, although it is more a derivative of metal-air batteries (e.g. Zn-air). A schematic representation of the rechargeable non-aqueous Li-O2 cell is shown in Fig 1. On discharge, lithium ions formed at the lithium metal anode are transported across the electrolyte and into the pores of the air-cathode. O2 from the atmosphere enters the cathode, and dissolves into the electrolyte within the pores. It is then reduced at the porous carbon electrode surface by electrons from the external circuit and combines with Li+ from the electrolyte, leading to the formation of solid Li2O2 as the final discharge product. Surprisingly, the reaction is reversible, Li2O2 can be oxidised, releasing oxygen gas, thus making this an energy storage device: 2Li + O2 ↔ Li2O2.
The challenge for the Li-O2 cell is the progress of development of the air-cathode that allows reversible formation of Li2O2 in a stable electrolyte within its pores [2-4]. A typical air cathode comprises of a carbon black mixed with a polymeric binder.
|
| The porous air cathode is required to accommodate the insoluble discharge product (Li2O2) as well as to facilitate oxygen diffusion to the reaction site through the cathode film. In addition, the porous carbon should provide sufficient network conductivity to deliver electrons to the reaction site efficiently with little overall impedance. A homogenous distribution of nano-sized catalyst (commonly either a noble metal or a transition metal oxide) may also be required to maximize the performance by increasing the round-trip efficiency by lowering the voltage gap between charge and dis-charge processes. This presentation will provide an overview of current developments in non-aqueous Li-O2 cells, followed by discussion of recent data on the role of the electrolyte and catalysts in these systems. |
 |
|
|
|
1. P.G. Bruce, S. Freunberger, L.J. Hardwick, J.-M. Tarascon, Nature Mater. (2012) 11 19
2. S. Freunberger, Y. Chen, N. Drewett, L.J. Hardwick, F. Bardé, P.G. Bruce,
Angew. Chem. Int. Ed. (2011) 50 8609
3. S. Freunberger, Y. Chen, Z. Peng, J. Griffin, L.J. Hardwick, F. Bardé, P. Novák,
P.G. Bruce, J. Amer. Chem. Soc. (2011) 133 8040
4. Z. Peng, S. Freunberger, L.J. Hardwick, Y. Chen, V. Giordani, F. Bardé, P. Novák,
J.-M. Tarascon, D. Graham, P.G. Bruce, Angew. Chem. Int. Ed. (2011) 50 63514. V
|
|
| < volver |
|
| |
|
|
|