| |
 |
| |
| Selecting and Designing Carbons for Li-Air Battery applications |
|
Peter Hall
Department of Chemical and Biological Engineering
University of Sheffield, Sheffield, UK |
| |
The reversible chemical reaction at the positive electrode for Li-air batteries is a complex three-phase reaction in which Li+ interacts with O2 on the surface of a catalyst loaded porous carbon in the presence of a liquid electrolyte, not forgetting the need for an electron.
Every aspect of this reaction is the subject of concentrated research effort and this paper focuses on the nature of the carbon component of the battery. The ideal carbon shares many features in common with carbons used as catalyst supports for other heterogeneous chemical reactions. An appropriate pore size distribution and large specific surface area to permit the electrochemical reaction to take place is of primary importance, as is chemical stability.
Additional requirements for carbons for Li air batteries area include a large pore volume, since the discharge product seems to fill the porosity rather than form a monolayer. Good electrical conductivity is also required to minimise Ohmic losses.
|
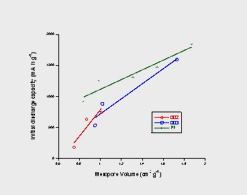 |
Our research has focussed on two kinds of carbon; resorcinol formaldehyde (RF) and phenol formaldehyde (PF) derived carbons. These materials have the advantage of being electrically conductive and have pore structures that can be controlled during synthesis, drying and subsequent activation of the carbon. Our investigations have shown that the initial discharge capacity of Li-O2 batteries correlates well with mesopore volume as derived from N2 adsorption data. This is summarised in the Figure, which shows the correlation between mesopore volume and initial discharge capacity. The PF line represents data for a PF-derived carbon activated under CO2 to different levels, while the 002 and 003 lines represent two RF-derived carbons also activated under CO2 at different temperatures. |
|
| |
| This seems to argue that mesopore volume should be maximised to increase capacity, however, the data points clustered around 1 cm3g-1 show that mesopore volume is not the only capacity determining factor. No simple pore characterisation (such as mean pore size) explains this variation. There is a further complication in understanding the role of porosity regarding cycling. Although PF-derived carbon electrodes exhibit greater initial discharge capacities, they tend to lose capacity on cycling much faster than the corresponding RF-derived carbons. This may be associated with the accumulation of discharge product in more highly restricted mesopores that is not easily broken down during the charging process. |
| |
| Acknowledgements This work was supported by EPSRC grant number EP/D031672/1. |
|
| < volver |
|
| |